rave etmf|Rave eTMF Systems : Baguio Rave eTMF の直感的で簡素化されたユーザー インターフェイスは、包括的な検索 . 238K Likes, 816 Comments. TikTok video from Siarly ♡ (@siarlyxo): “Vieron el perrito? 🐶”. siarly. Surround Sound - JID.Bais City Tourism: Tripadvisor has 392 reviews of Bais City Hotels, Attractions, and Restaurants making it your best Bais City Tourism resource.
rave etmf,Rave eTMF (electronic Trial Master File) is a collaboration platform that streamlines creating, managing, and populating clinical trial content. Rave eTMF simplifies clinical document filing .
Rave eTMF の直感的で簡素化されたユーザー インターフェイスは、包括的な検索 .
Rave eTMF Systems 메디데이터 플랫폼에 통합된 Rave eTMF는 TMF 및 주요 artifact를 Rave CTMS에 .
Rave eTMF is a global, secure collaboration platform to seamlessly manage Trial Master File content so it is always contemporaneous with the study. Rave eTMF streamlines content .Rave eTMF Simplify your TMF Medidata Rave eTMF makes overseeing clinical trial artifacts simple. Unified with Rave, eTMF powers trials to run faster, lowers risks managing regulated .Peb 5, 2024 — With full support for the latest versions of the TMF Reference Model, eTMF Vault gives sponsors and CROs real-time access to clinical documentation at every point in a trial’s .
Learn how Medidata's Rave CTMS and Rave eTMF, unified on the Medidata platform, streamlines clinical operations workflows and accelerates study start-up by combining content .Rave eTMF – Simplify Trial Oversight with Unified Document Management. By Medidata. Enter your details to receive the free white paper: Your Trial Master File (TMF) complexity is .Hun 5, 2023 — Rave eTMF is a global, secure collaboration platform to seamlessly manage Trial Master File content so it is always contemporaneous with the study. Rave eTMF streamlines .Okt 14, 2021 — Rave CTMS and eTMF are key offerings within Medidata’s Unified Platform, a cutting-edge platform that is transforming the clinical trial experience for patients, sponsors, .Because Rave eTMF is unified with the Medidata Unified Platform, study teams can manage TMF content seamlessly and accurately while maintaining inspection readiness and compliance. .
Dive deep into the online self-paced, flexible, Trial Master File course. Elevate your eTMF knowledge and skills with our specialized guidance.

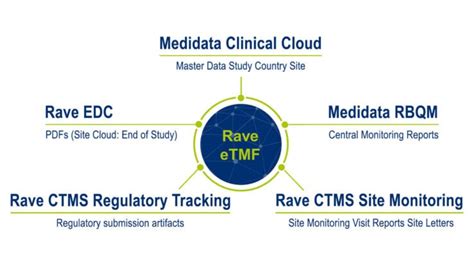
Rave와 통합된 eTMF는 임상시험이 빠르게 진행될 수 있도록 하고 규제 문서의 관리 위험을 낮춰주며 적은 리소. 스로도 복잡성을 줄임으로써 높은 품질의 실시간 데이터 관리를 제공합니다. 의료기기 기업, 임상 담당 부사장 Medidata Rave eTMF + Rave EDCRave eTMF可通过整合整个研究生命周期中的内容和数据来简化临床文件归档流程。作为Medidata Clinical Cloud ® 的一部分,Rave eTMF能够即时自动填充平台上其他应用程序的内容和数据,保证您的TMF始终完整。 生成新的研究计划仅需几分钟,同时支持用户自定义文件计划。Ago 1, 2023 — Having integrated Rave eTMF and Rave CTMS, Medidata’s platform eliminates the need for IT resources to manage the solution. This allows CTMS and eTMF to work together with bidirectional data transfer, offering comprehensive trial oversight and ensuring accurate document compliance. This integration connects study teams to coordinate and .Rave eTMF is a global, secure collaboration platform that easily manages Trial Master File content so it is always contemporaneous with the study. Learn more. Medidata Detect Medidata Detect is a powerful data and risk surveillance tool for centralized monitoring, powered by automated statistical algorithms and machine learning. Detect improves .

Rave CTMSは、Rave EDCおよびRave eTMFと緊密に統合されており、現場とのコラボレーションを最適化します。ユーザーは一度文書をアップロードすれば、eTMFは自動的にデータとコンテンツを更新します。
Medidata Rave eTMF 使临床试验数据录入的监查变得简单。与 Rave 系统相统 一的 eTMF 能够加快试验进度,并通过管理受监管的数据,降低试验风险,而且, Rave eTMF 在提高实时数据管理质量的同时,还能减少所需资源并降低操作复 杂性。 目前最灵活、直观的 eTMF 解决 .Improve speed and efficiency for the oversight of studies with Rave CTMS (Clinical Trial Management System). eTMF. Manage electronic trial master file content while maintaining readiness & compliance. Clinical Trial Financial Management; Site Payments. Unify your clinical research study data and financial management in one platform. Grants Manager
Rave EDC offers you fast implementation and maximum control to support clinical trials. Our flexible EDC system empowers clinical research teams to capture, cleanse, and manage study data, ensuring efficient trial execution across every phase of the clinical trial life cycle. . eTMF. Manage electronic trial master file content while .
Rave와 통합된 eTMF는 임상시험이 빠르게 진행될 수 있도록 하고 규제 문서의 관리 위험을 낮춰주며 적은 리소. 스로도 복잡성을 줄임으로써 높은 품질의 실시간 데이터 관리를 제공합니다. 의료기기 기업, 임상 담당 부사장 Medidata Rave eTMF + Rave EDC
rave etmfRave CTMS 및 RAVE eTMF에는 시험 개시 관련 이벤트들이 포함되어 있으며, 시험기관 별 필수 주요 일정과 작업을 한 곳에서 추적할 수 있습니다. 시험기관 활성화 활동 및 문제 관리를 위한 폐쇄 루프(closed-loop) 시스템은 다수의 상태 스프레드 시트 및 추적 리포트를 .利用 Rave CTMS(临床试验管理系统)提高研究监督的速度和效率。 eTMF. 管理电子临床试验主文档的同时,保证合规性,并为审查做好准备。 Grants Manager 试验金融管理. 快速、准确地编制研究人员补助金预算。 Site Payments 研究中心付款With Rave eTMF, streamline and simplify TMF content audit-readiness. Read the infographic to learn how easy-to-use Rave eTMF streamlines creating, managing, and populating clinical trial content while providing full support for the Drug .Rave eTMF. MEDIDATAが提供する「Rave eTMG」は単体でも利用できますが、同社の他システム(CTMS、EDCなど)と連携することで、承認プロセスの管理など、治験や臨床試験に関するコンテンツの作成・管理・収集をどんどん合理化することができます。 Rave eTMFのMay 9, 2020 — 메디데이터 Rave eTMF는 임상시험의 문서 수명 주기의 관리를 간소화합니다. Rave와 통합된 eTMF는 임상시험이 빠르게 진행될 수 있도록 하고 규제 문서의 관리 위험을 낮춰주며 적은 리소스로도 복잡성을 줄임으로써 높은 품질의 실시간 데이터 관리를 제공합니다.통합 된 eTMF 메디데이터 Rave eTMF 솔루션은 Archive, CTMS, RBQM, Payments 및 Rave EDC를 포함한 메디데이터 Rave Clinical Cloud와의 통합을 통해 업계에서 가장 동적이고 포괄적인 실시간 end-to-end TMF를 제공합니다. Rave eTMF를 이용하면 시험기관 검증 방문,Peb 5, 2024 — eTMF is a cloud-based electronic trial master file system that enables organizations to efficiently manage their clinical trials. With full support for the latest versions of the TMF Reference Model, eTMF Vault gives sponsors and CROs real-time access to clinical documentation at every point in a trial’s set-up, execution, and archival.Improve speed and efficiency for the oversight of studies with Rave CTMS (Clinical Trial Management System). eTMF. Manage electronic trial master file content while maintaining readiness & compliance. Clinical Trial Financial Management; Site Payments. Unify your clinical research study data and financial management in one platform. Grants ManagerMedidata Rave eTMF + Rave EDC. Simplify your TMF . Medidata Rave eTMFmakes overseeing clinical trial artifacts simple.Unified with Rave, eTMF powers trials to run faster, lowers risks managing regulated content and delivers higher quality real-time data management while requiring fewer resources and reducing complexity.
rave etmf|Rave eTMF Systems
PH0 · eTMF Overview
PH1 · Trial Master File Course eTMF experience Certificate
PH2 · TD2 Partners with Medidata to Enhance its Clinical Operations
PH3 · Rave eTMF “Efficiently maintaining and extracting both data and
PH4 · Rave eTMF – Simplify Trial Oversight with Unified Document
PH5 · Rave eTMF Systems
PH6 · Rave eTMF Fact Sheet: Simplify Trial Oversight with Unified
PH7 · Rave CTMS & Rave eTMF
PH8 · Medidata Rave eTMF (Trial Master File) Fact Sheet